New Cellular Therapy Restores Damaged Cornea in 14 Blind Patients
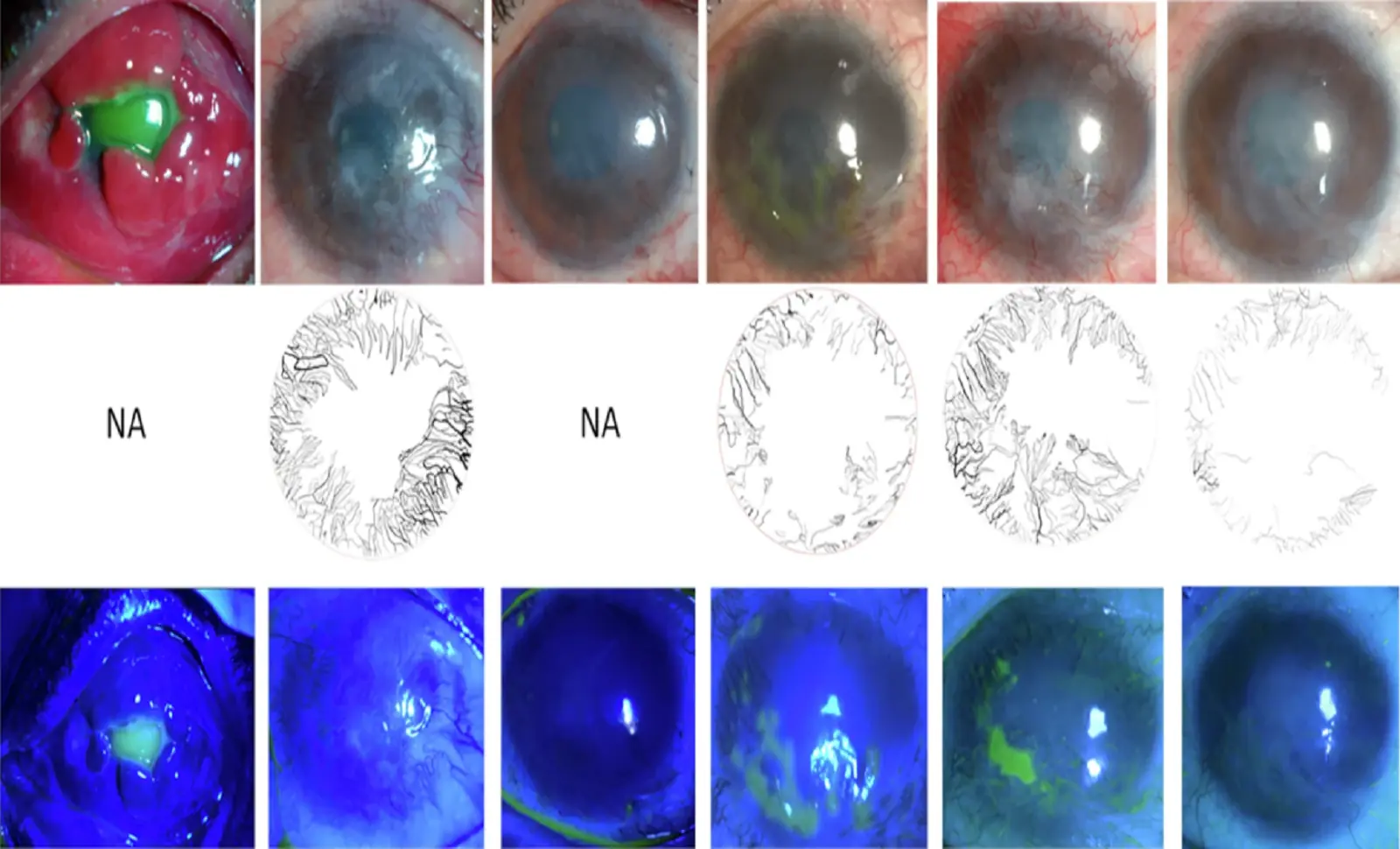
Limbal stem cell deficiency (LSCD) is a condition that affects many people worldwide. This occurs when the stem cells located in the limbus—the outer part of the cornea—stop functioning properly. As a result, the cornea loses its ability to regenerate, leading to inflammation, scarring, and, in severe cases, vision loss.
Researchers at Massachusetts Eye and Ear have developed an innovative cell therapy called Cultivated Autologous Limbal Epithelial Cell (CALEC) transplantation, which has shown promising results. In a clinical trial with 14 patients, this groundbreaking technique achieved a 92% success rate, offering hope to those suffering from this debilitating eye condition.
What is CALEC Therapy?
The CALEC procedure is based on extracting and cultivating limbal epithelial stem cells from the patient’s own healthy eye. Doctors take a small tissue sample from the healthy eye and cultivate the cells in a laboratory-free of xenobiotics (foreign substances), serum, and antibiotics.
Once these cells have multiplied, they are transplanted onto the damaged cornea to restore its surface and improve vision. This allows the eye to heal and regenerate healthy tissue, something previously impossible for patients with severe LSCD.
Read More: Groundbreaking Gene Therapy Restores Vision in Four Children with Congenital Blindness
Study Results
The Phase I/II clinical trial evaluated the safety and effectiveness of CALEC therapy in 15 participants, with 14 successfully completing the study. The findings were highly positive:
✅ 93% of cultivated cell grafts met quality criteria for transplantation.
✅ 86% of patients showed improved corneal integrity within 3 months.
✅ 93% achieved partial or complete vision restoration within 12 months.
✅ 92% maintained positive results after 18 months.
Additionally, no severe complications such as infections or transplant rejection were reported. Only one case of bacterial infection occurred, but it was unrelated to the treatment.
Why Is This Therapy a Major Breakthrough?
Before this study, treatment options for limbal stem cell deficiency (LSCD) were limited. Many patients attempted corneal transplants, but these often failed since the eye could not regenerate properly.
With CALEC, the patient's own tissue is used, significantly reducing the risk of rejection and adverse effects. Additionally, this is the first U.S.-based study to eliminate animal-derived serum and antibiotics, making it safer and more compatible with the human body.
Future Implications
While these results are promising, researchers emphasize the need for further clinical trials to confirm the treatment’s effectiveness on a larger scale. If future studies continue to demonstrate success, CALEC therapy could become the standard treatment for individuals suffering from severe LSCD.
This breakthrough may also pave the way for new stem cell therapies to treat other eye diseases and skin conditions.
Conclusion
Blindness caused by limbal stem cell deficiency has been a challenging condition to treat for decades. However, CALEC transplantation therapy is now offering new hope to patients who have lost their vision due to this disease.
With a 92% success rate in its initial clinical trial, this regenerative therapy has proven to be both safe and effective in restoring corneal health. While more studies are needed to confirm its long-term benefits, this research marks a significant step forward in using cellular therapies for treating eye diseases.
This groundbreaking medical advancement could transform the lives of thousands suffering from corneal blindness, while also laying the foundation for future applications of regenerative medicine in various health fields.
News in the same category

New Cellular Therapy Restores Damaged Cornea in 14 Blind Patients

10 Ways to Help Reduce Your Colon Cancer Risk

7 Signs Of Intestinal Parasites Living Inside Your Body
20 Cancer Signs People Ignore Until It’s Too Late

Doctor makes worrying claim that ‘every new patient’ at cancer clinic is under 45 and blames one thing

10 Alarming Signals Your Body Might Be Warning You About
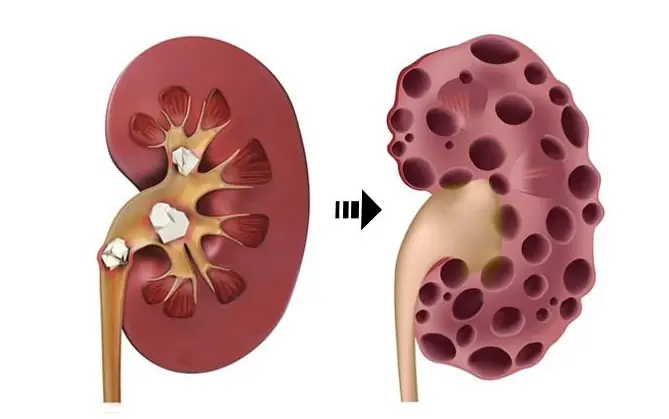
KIDNEY DISEASE SILENTLY CREEPS IN – IF YOU NOTICE THIS AT NIGHT, BEWARE!
5 Reasons to Have Protein Before Bed

The Tiger Spider: Nature’s Defense Against the Violin Spider

9 Foods to Consider Limit if You Have Hypothyroidism
Attention, Parents! You Might Want To Hold On To Your Kids’ Baby Teeth

10 Ways Baths Can Be Either Good and Bad for Your Body
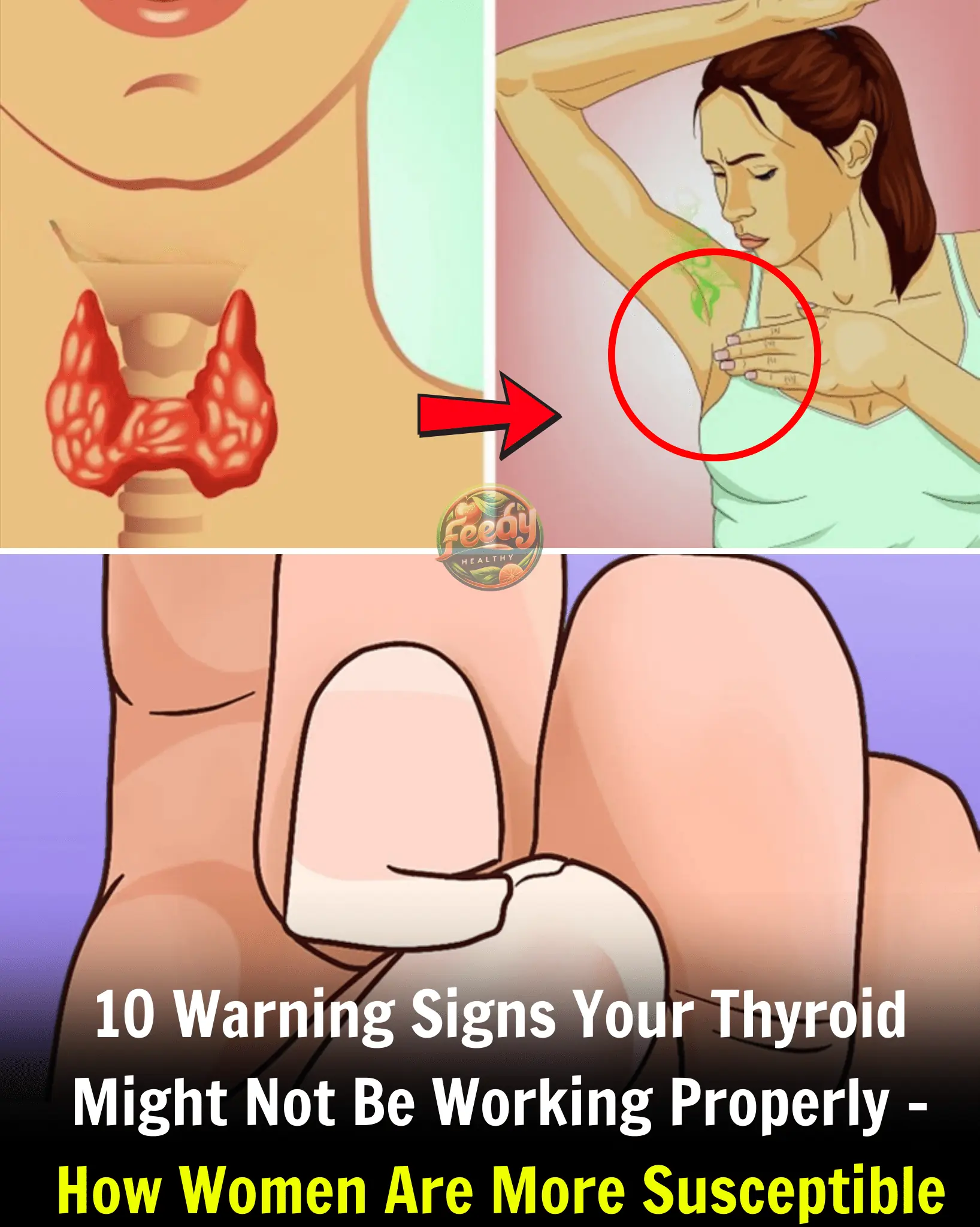
10 Warning Signs Your Thyroid Might Not Be Working Properly
What Happens After 30 Days of Cold Showers

A Groundbreaking Medical Breakthrough Brings Hope for the End of HIV/AIDS
5 Alarming Stroke Warning Signs to Watch for in Young People
Peeing in the Shower, Doctor Explains Why Women Shouldn’t
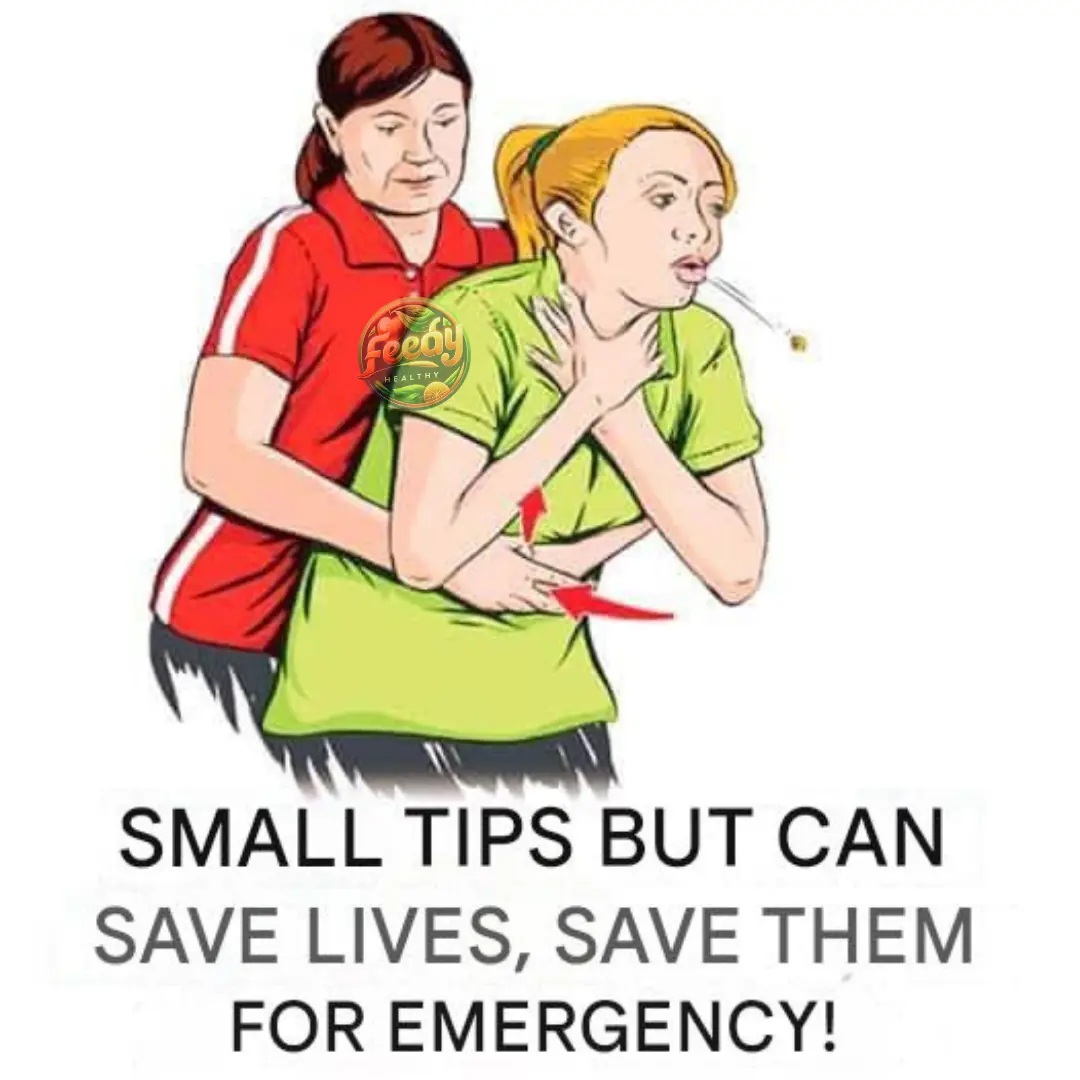
A small trick that can save lives.
News Post

PUT OIL ON THE SOLES OF YOUR FEET

10 Ways to Help Reduce Your Colon Cancer Risk

Persistent Symptoms After the COVID-19 Vaccine: New Study Reveals Unexpected Immune Effects

Secrets to Youthful, Radiant Skin for Women Over 40

Rosemary for Leg Pain: Natural Relief for Joints and Back

Mix Vaseline with Lemon – You’ll Be Amazed by the Results!

The conductor seated the pregnant stowaway in the compartment with the strange old man. At night, screams echoed through the car.

7 Signs Of Intestinal Parasites Living Inside Your Body
Forget Pills: Natural Remedies for Effectively Treating Thyroid Disorders

Why Do You Have Bleach Stains on Your Underwear? The Surprising Truth

Refreshing Carrot, Lemon/Lime, and Apple Juice Recipe

New Father Kicks Wife With Newborn Twins onto the Streets, Years Later He Begs Her for Help

20 Cancer Signs People Ignore Until It’s Too Late

Doctor makes worrying claim that ‘every new patient’ at cancer clinic is under 45 and blames one thing

Why hang a clothespin on the shower?

Effects of smartphone restriction on cue-related neural activity

10 Alarming Signals Your Body Might Be Warning You About

KIDNEY DISEASE SILENTLY CREEPS IN – IF YOU NOTICE THIS AT NIGHT, BEWARE!
